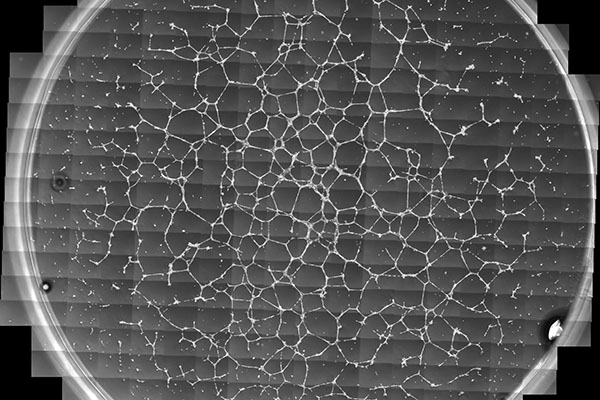
The research team of the Cleveland Cord Blood Center is exploring the application of umbilical cord blood-derived induced regulatory T (iTreg) cells for the prevention and treatment of diabetic retinopathy.
In patients with this condition, the reduction in the patients’ original Treg cells leads to a cycle of inflammation and abnormal growth of blood vessels in the retina. As the inflammatory response increases unchecked, abnormal blood vessel growth occurs, causing additional inflammation – a destructive cycle for individuals with either Type 1 or Type 2 diabetes. Eventually, cumulative damage disrupts light-sensitive tissues in the retina, leading to a loss of vision.
The two existing treatment protocols for diabetic retinopathy target one of these factors but not both. The inflammatory response can be treated with injections of steroids while abnormal blood vessel growth can be treated with antibody injections. Both of these treatment protocols require follow-up injections into the eye every 30 to 90 days, not a pleasant experience for patients.
The cord blood induced iTreg cell injection shows promise by suppressing both the inflammatory and abnormal blood vessel formation components of this disease. Rather than receiving the antibody or steroid injections every 30 to 90 days, iTreg cells could establish themselves locally in the tissue. They expand appropriately when inflammation occurs and reduce once the inflammation subsides, meaning a single injection might be sufficient.
The research shows early positive results, an indication that a new iTreg injection would address both the inflammation response and abnormal blood vessel growth found in diabetic retinopathy. The prospects of an improved standard of care look very promising.
CCBC’s commitment to finding new cell therapy solutions in this field is well aligned with the organization’s mission of advancing umbilical cord blood cell therapy treatments to save lives, enhance health and expand knowledge, one birth at a time.


