TAKING THE LIFESAVING WORK OF CORD BLOOD TO THE NEXT LEVEL
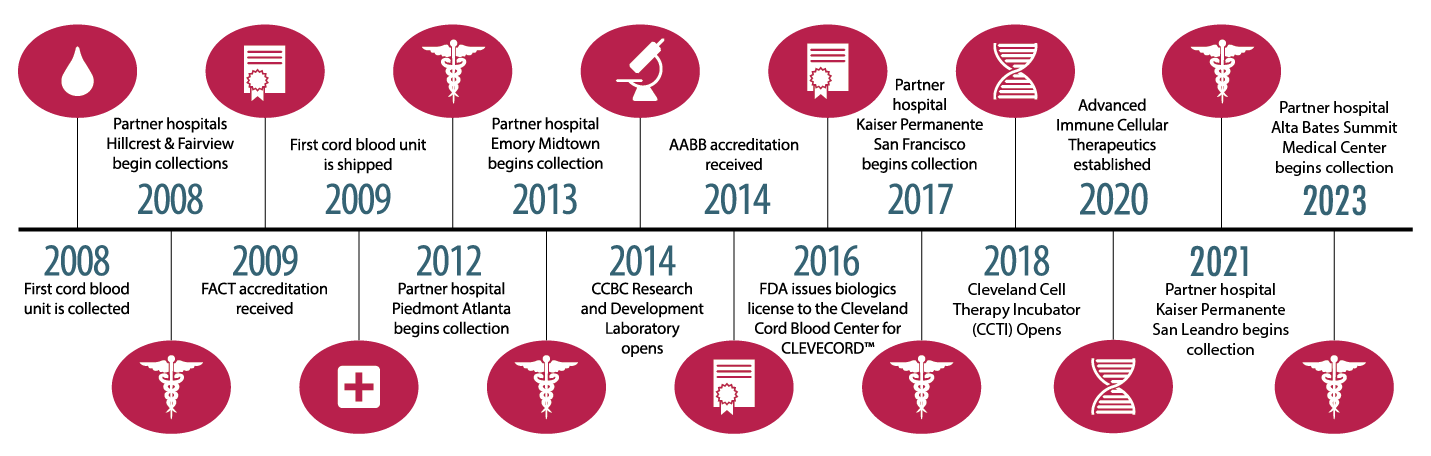
Mary J. Laughlin, M.D., established the Cleveland Cord Blood Center (CCBC) in 2008 in conjunction with the growing role of stem-cell rich umbilical cord blood for transplantation in patients with life-threatening disorders such as leukemia, lymphoma and immune system disorders. Dr. Laughlin, who performed one of the world’s first successful umbilical cord blood stem cell transplants on an adult leukemia patient in 1995, launched the public cord blood bank with the commitment to serve the unmet needs of a diverse population.
Since its establishment, the Cleveland Cord Blood Center has become:
- A leader in the collection, processing, storage and distribution of quality cord blood stem cell units
- A researcher in the exploration of umbilical cord blood’s expanding potential as a cell therapy treatment
- An innovator in the field of umbilical cord blood cellular therapy initiatives
- A prominent player in fostering greater awareness of the life-giving and lifesaving potential that cord blood stem cells hold
FAST FACTS
The Center has provided more than 16,000 research-grade units to investigators at partner organizations including Stanford University, Canventa Life Sciences, Case Western Reserve University, Cleveland Clinic Foundation, Global Cardiovascular Innovations Center, Centers for Disease Control & Prevention, Indiana University, Lonza RTP, StemExpress, LLC, University of Louisville, West Virginia University, Boston Children’s Hospital, Cellenkos, Inc., Gamida-Cell, Ltd., Jackson Labs, OnK Therapeutics, and Deverra Therapeutics, Inc.
FDA APPROVAL FOR CLEVECORD™
On September 1, 2016, the Cleveland Cord Blood Center received a biologics license from the U.S. Food and Drug Administration (FDA) for CLEVECORDTM, a stem cell product derived from umbilical cord blood for use in stem cell transplants. The FDA approval of CLEVECORD reflects the organization’s ability to meet the highest quality standards in the industry for distribution of cord blood products to transplant centers throughout the U.S. and around the world.
SOCIAL ENTERPRISE SUBSIDIARIES LAUNCHED
In the advancement of the Cleveland Cord Blood Center’s mission, the Center established two nonprofit social enterprise subsidiaries in 2020. The Cleveland Cell Therapy Incubator (CCTI) and Advanced Immune Cellular Therapy (AICT) will carry on the next generation of the Center’s lifesaving work with an income strategy integrated into the high-level social mission. These subsidiaries will help enable the Center to develop advanced cellular therapies as well as provide access to additional sources of funding.